 It is understood that currently there are three types of contact lenses on the market: First, regular imported products or domestic brands, there are regular agents, the price is more expensive, such as Johnson & Johnson, Haichang, etc.; second, a large number of non-formal channels imported Korean brands or Japanese brands, quality is difficult to judge; Third, low-priced brand-name products, mostly from small workshop production, the purchase price of only a few dollars.
It is understood that currently there are three types of contact lenses on the market: First, regular imported products or domestic brands, there are regular agents, the price is more expensive, such as Johnson & Johnson, Haichang, etc.; second, a large number of non-formal channels imported Korean brands or Japanese brands, quality is difficult to judge; Third, low-priced brand-name products, mostly from small workshop production, the purchase price of only a few dollars. An industry source told reporters that the current production process of colored contact lenses, there are three kinds of pigment printing, pigments, laminated film manufacturing. Brands with high reputation for contact lenses use pigments that are commonly known as "sandwich" technology. The process divides the lens in three layers and sandwiches the color film in two layers. Due to the relative safety, most color contact lens products use this process and the cost is relatively high. However, some small workshops mostly use pigment printing technology, that is, paint directly on the inside of the lens, and then baked to make the color easily penetrated, causing great harm to the eyes.
"Illegal production of these contact lenses costs only about two or three yuan, plus the printing of trademarks, packaging, etc., the cost will not exceed 10 yuan." The source revealed.
Contact lens lot number confusion
In practice, the supervision of contact lenses is mostly limited to the examination of production and business qualifications, and it is relatively weak in specific aspects. For example, after some enterprises have obtained production or sales qualifications, their production environment is declining; some directly purchase and sell counterfeit products, etc. Although the country has incorporated contact lenses into the supervision of the third type of medical devices, the reporter visited and found the approval document. The use of the number is very confusing.
The reporters entered the two batch numbers on the official website of the State Food and Drug Administration and found that the former had the Chinese brand name “Bao Nuo†and the after-sales agency was changed from “Beijing Haihua Han Shi Bao Trading Co., Ltd.†to “Shanghai Ji Yi’ao. Optical Co., Ltd.", date of change is February 20, 2010, and the expiry date is September 10, 2013. The latter has no Chinese trade name. The product name column reads "Soft Contact Lens" and the agent The person and after-sales agency was changed from "Shanghai Jiyiao Optics Co., Ltd." to "Shanghai Gaolaobo Optics Co., Ltd." The change date was April 3, 2013 and the expiry date was December 8, 2015.
Although there is a relationship between the two, but many businesses have used this "empty", not only the two batches of products at the same time in the sale, but also use the product lot number of the mold area, directly in other commodity bottle Paste the product "label", but the batch number is not the above two batch numbers, and some even use its logo, the entire bottle is Korean. For example, the online “Yimei Simei Yun series USP color contact lens half a year tossing 1 pieceâ€, “Misseye Keli Meng light large-diameter US pupils annual throwing contact lenses†are approved in the product parameter column are written on the number "The National Food and Drug Administration (Int.) Word 2009 No. 3222028", but it is not an agent issued by Shanghai Jiyi Optical Co., Ltd.
Not only did the lot number exist in the model area, the reporter also discovered that some contact lenses were serial numbers, or several products used the same batch number. In the shop of Guangzhou Glasses City, two brands of contact lens products named “Shui Ling Ling†and “Chun Run†use the same approval number: “National Food and Drug Administration (Jin) 2012 2012 No. 3220229â€. The reporter did not find the approval number on the official website of the National Food and Drug Administration.
At the same time, the products that claim to possess the "National Food and Drug Administration (Jin) Machinery 2012 No. 3220222" batch number have products such as "Enni Love" and "Aime Audrey", but this lot number is in the State Drug Administration. The corresponding product name in the database of the Directorate General is "VENUS EYES".
In fact, relevant departments have already noticed the "flooding" of poor contact lenses.
In March last year, the State Food and Drug Administration issued a notice requesting that, as of April 1, 2012, no medical device registration certificate for decorative colored plain contact lens products, and corresponding production and operation qualification certificates, be produced and Operating such products.
However, in practice, supervision of contact lenses is mostly limited to the examination of production and operating qualifications, and it appears to be weak in specific aspects. For example, after some enterprises have obtained production or sales qualifications, their production environment is declining; some directly purchase the goods and sell counterfeit products.
"Contact lenses directly contact the eyes, in addition to the bacteria will cause harm to the human body, the color of the counterfeit color lens plated without strict treatment, will induce keratitis, corneal ulcers and other eye diseases, more serious is the lens Once the color material falls off and adheres to the eye, it may even cause blindness.†An associate professor of corneal diseases at the Eye Hospital of Sun Yat-sen University told the reporter that the number of cases of eye diseases caused by inappropriate wearing of colored contact lenses has increased significantly in the past two years. The trend is that patients are mostly young people.
The expert suggested that administrative law enforcement agencies that have supervision functions for contact lenses should strengthen daily linkages. In addition to the production process, they must also jointly fight the entire chain of counterfeiting and sales fraud to protect consumers' "eyeballs." At the same time, he advised young people not to “be cheap and cheapâ€. When buying contact lenses, they must go to the eyewear sales shop of the joint venture and choose suitable glasses according to their personal circumstances.
Cleaning Engineering For Hospital
Cleaning Engineering For Hospital
The medical clean engineering system covers clean operating rooms, ICUs, disinfecting supply rooms, laboratories, clinical laboratories, vein deployment centers and other sites. Decoration materials, clean air-conditioners and medical devices that meet the cleanliness requirements must be adopted to ensure cleanliness, especially for clean operating rooms, ICUs, disinfecting supply rooms and other sites subject to cleanliness requirements of the hospital.
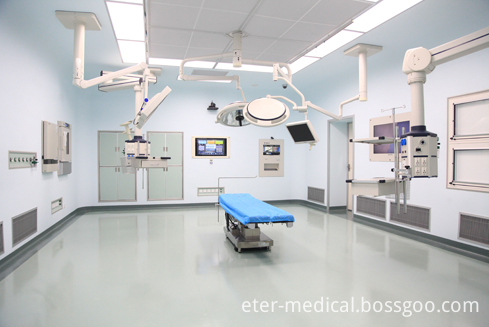
Cleaning Engineering For Hospital,Environmental Services Hospital,Clean Operation Theatre Engineering,Medical Air-Cleaning Engineering
Hunan Eter Medical Co., Ltd. , https://www.eter-tech.com