--editor
After being dried with vermicelli, it is colored into Cordyceps sinensis and smoked with sulfur to make the yellow peony white. These are not invented, but are happening on the national herbal medicine market. Wang Yishuo, a graduate student from Henan University of Traditional Chinese Medicine, complained to the reporter that every time the college uses Chinese herbal medicines for experimental purposes, it will encounter “Li Guiâ€. This probability is about 30%. Even if they like these professional medicines, they will inevitably be “invited.â€
In fact, in the tide of rising prices of Chinese herbal medicines, driven by profits, the phenomenon of counterfeiting and adulteration of Chinese herbal medicines has intensified, and Chinese Herbal Pieces are under siege of counterfeit goods. Although the state has already made it clear that major medicinal herbs markets are not allowed to operate Chinese herbal medicines, there are still quite a lot of herbal medicines in the market.
Why black drugs in the herbal market continue to be prohibited? Where does its gray chain extend?
The reporter learned from the recently concluded “2010 Chinese Medicine Industry Summit Forum†that with the promulgation of the 2010 edition of the Pharmacopoeia and the completion of the revision of the new GMP, the State Food and Drug Administration (SFDA) has drafted two documents and tried to It is strictly forbid that both fake medicines and black-decoction tablets will flow into the pharmaceutical production field in two aspects: the supervision of raw material procurement by Chinese medicine production companies and the strengthening of management of Chinese herbal medicines. The two documents will be issued as ancillary documents after the release of the new Pharmacopoeia and the new GMP.
According to industry sources, many policies in the TCM decoction industry are poised for future development. In the future, the “Great Governance†environment will be created. There will only be two roads in front of the production companies. They will either actively improve product quality to meet the requirements of the new standards or end up standing still. Was eliminated.
The status quo is worrying
According to the relevant regulations of the country, Chinese herbal medicines belong to agricultural and sideline products, and farmers can sell on the professional market. The Chinese Herbal Medicine Pieces are medicines that cannot be processed and sold by farmers. They must be produced by qualified Chinese herbal medicines. However, as of the end of September 2010, there were a total of 891 TCM decoction pieces that had passed GMP certification in China. However, more than 300 companies actually started production. However, more than 500 companies did not withdraw from the market. Some of them relied on product rebranding. While alive, others do abnormal businesses such as “take tickets†and “attachmentsâ€. Specifically, “OEM†means that production companies do not produce Chinese Herbal Pieces and purchase directly from “workshops†in the Chinese herbal medicine market, and affix the company’s brand; “hanging†is an unscrupulous trader affiliated to a qualified company, while enterprises rely on rental production. Licensing and production equipment are alive; "take tickets" companies survive illegally buying and selling bills. In the circulation field, some pharmaceutical and commercial companies also play a disgraceful role. They arbitrarily purchase pieces of decoction produced by small workshops from the herbal medicine market, and sell fake counterfeit products. “These illegally produced and operated enterprises and the small workshops in the herbal medicine market have formed an invisible grey supply chain, which is extremely harmful to the Chinese Herbal Pieces Market,†said an industry source.
There are currently 17 professional medicinal markets in China. After the "SARS" in 2003, the country made great efforts to rectify the professional market of Chinese herbal medicines. Drug markets such as Zhangzhou and Anguo were closed down, which to a certain extent hit the "workshop" decoction pieces. However, since the drug market has a long history of medicinal planting and processing, it has naturally formed an upstream-downstream relationship. Many transactions are carried out in private, very secretive, and supervision is very difficult.
According to Zhang Shichen, director of the Chinese Herbal Medicine Professional Committee of the Chinese Medicine Association, there are three main reasons for the formation of this situation: First, there is not enough efforts to rectify the professional market of Chinese herbal medicines, and there is local protectionism; second, the standards for concocting traditional Chinese medicine decoction pieces are not uniform and regional market drugs are used. Habits have led to serious protection of the local market, it is difficult to form large-scale enterprises, the industry concentration is poor; Third, the detection standards of Chinese Herbal Medicine Pieces are relatively low, and there has been a long-standing misunderstanding of the importance of appearance.
Statistics show that in 2009, the output value of the Chinese medicine decoction pieces industry has exceeded 50 billion yuan, accounting for 19.2% of the total output value of the Chinese medicine industry, an increase of 26.5%; the total profit of 3.3 billion yuan, an increase of 40.3%. Chinese Herbal Medicine is the fastest growing field in the medical sub-sector. However, the export of TCM decoction pieces has frequently encountered returns from the international market and has been plagued by problems such as excessive heavy metals and residual sulfur dioxide, which has seriously affected the international image of TCM products.
"Great rule" is coming
According to Fang Shuting, president of the Chinese Medicine Association, for a long time, the lack of a correct understanding of traditional Chinese medicine decoction pieces has led to the vagueness of Chinese herbal decoction pieces, which is a deep-seated reason for market confusion. "Chinese medicine pays attention to syndrome differentiation, and Chinese Herbal Medicine is a prescription drug prescribed by traditional Chinese medicine. This has been neglected for a long time. As a prescription drug, China has a strict system of examination and approval of pharmaceutical production and production practices. It is an illegal act to sell Chinese Herbal Medicine Pieces by unauthorized processing. The "National Essential Drugs Catalogue" released last year contained Chinese Herbal Pieces for the first time. This is a state's emphasis on Chinese Herbal Pieces and it is also a powerful illustration of its use as a prescription medicine."
The reporter noticed that compared with the 2005 edition of the Pharmacopoeia, the 2010 edition of the Pharmacopoeia not only significantly increased the number of Chinese Herbal Pieces, but the important thing was that for the first time, it was clear that the definition of the “decoction†means that the herbs can be directly used in the clinical or preparation production of traditional Chinese medicine after concoction. "Prescription drugs", for the first time, clarified that all drug-seekers were decoction pieces and treated "medicinal materials and decoction pieces" as an independent category.
SFDA officials said at the forum that the 2010 edition of the Pharmacopoeia and the upcoming release of the new version of GMP have comprehensively improved the standards for Chinese herbal medicines. Two supporting documents are needed to strengthen supervision in the new environment.
Judging from the two draft documents that the SFDA has solicited for corporate opinions, many articles refer directly to the market's ills. For example, the document requires that the Chinese Herbal Piece Company provide two test reports for the purchase of raw materials and the delivery of finished products. The former blocked false medicinal materials from entering the pharmaceutical production process, and the latter protected the finished product quality. For another example, due to the restrictions on the distribution of medicinal planting resources, there are often not more than a dozen species that are independently produced by traditional Chinese herb decoction pieces. In order to ensure local supply, many companies adopt the entrusted processing method to enrich the product line – and this is what breeds “OEMâ€. Non-standard behaviors such as “hanging†have led to the indiscriminate flow of bad-quality cut pieces into the market. In this regard, the documents stipulate strict regulations on commissioned processing of Chinese herbal medicines and pharmaceuticals.
Qian Zhongzhi, Director of the Standards Division of the China Pharmacopoeia Commission, said that the 2010 edition of the Pharmacopoeia significantly increased the effectiveness of Chinese medicines in identifying specific features that conform to the characteristics of traditional Chinese medicines, basically ending the history of “pill, powder, paste, dan, and immortalsâ€. : The number of TLC identification methods has been increased to 2,494 items, which reflects the peculiarity and accuracy of the new edition of pharmacopoeial Chinese medicine standards in ensuring the effectiveness; and the determination of effective, active, and multi-indicator components has increased the content of high-performance liquid chromatography 1138 Among the items, 713 items for effective active ingredient determination were accounted for, accounting for 62.7% of the total number of newly added items; 13 items of high-performance liquid chromatographs were included in the 2010 edition of the Pharmacopoeia, and 9 items were for fingerprints, making the overall method of controlling the quality of Chinese medicines The practical application has been greatly improved.
Active choice
A number of new policies are poised to take off, and the industry is changing. However, when the reporter and the company deeply contacted, they felt that there was still a big difference in the understanding of the production of Chinese herbal decoction pieces. Going left and going right, the enterprises were still hesitant.
A company official was outspoken: Although the new edition of the Pharmacopoeia was enacted in October, there are still very few companies that have the inspection capability required by the Pharmacopoeia nationwide, possess advanced detection equipment, and are able to do full inspections.
The relevant person of another company made it clear why. He said that due to the relatively small size and low profits of traditional Chinese medicine decoction pieces, it is reluctant to invest more than one million yuan in the purchase of testing equipment. At present, many companies without inspection ability want to achieve the pharmacopoeia standards through commissioned inspections.
In the symposium on the SFDA's request to strengthen the advice of companies on the supervision of Chinese herbal medicines, some companies believe that it is too cumbersome to request inspection reports on both the procurement of raw materials for raw Chinese medicines and the delivery of finished products; some companies hope that Chinese medicines Debris processing entrusted to leave a living, can not be a total ban. Sichuan New Lotus Chinese Herbal Pieces Co., Ltd. is the first company in China to pass GMP certification of Chinese Herbal Medicine, and is a leader in Chinese Herbal Pieces industry. In the past two years in the international market to improve the detection standards of Chinese medicine decoction pieces, the new Lotus products are recognized by South Korea, Southeast Asia and other countries and exported successfully, becoming the benchmark of high-quality Chinese Herbal Pieces. Jiang Yun, chairman of the company, stated that “the state’s strict control over TCM decoction pieces is a general trend. At present, the number of TCM Decoction Pieces is small and small, and with the improvement of decoction standards, it is bound to usher in the industry reshuffle. In this case, companies should If you want to continue to survive in this area, you only have to follow the trend and take the initiative to improve the quality of your products and meet the national standards. Whoever moves or walks ahead will seize market opportunities."
In fact, when many Chinese Herbal Pieces companies complain that “the national standard is too high to be reachedâ€, the Chinese Herbal Pieces industry has become a hot industry for capital pursuits. Li Lei, deputy director of the China Medicinal Insurance Chamber of Commerce's Herbal Pieces Division, said that many of the hot money that has flowed out of the real estate industry has already flowed into the Chinese herbal medicines and Chinese herbal medicines industry. According to monitoring, more than a hundred enterprises have been added this year alone. These enterprises have sufficient funds and all the standards required by the country have been achieved. This will be no small impact on the original Chinese Herbal Piece Company. "The survival of the fittest is the law of the market, but the influx of hot money has also formed a potential crisis. The profitability of hot money is destined to be impossible to have the belief of a hundred-year-old enterprise. If you earn one and leave, the damage to this industry will be great. Li Lei said.
The governance and transformation of the TCM decoction industry has been overwhelming. No matter if the company goes left or right, it is now time for the decision.
The Acupuncture Stimulator is a professional level acupuncture apparÂatus designed for detection of acupuncture points, massage (TENS), and for applying acupuncture (electro-needle therapy) and magneto-therapy.
The Acupuncture Stimulator a built-in timer and can output 5 different kinds of waveforms, each indicated by a symbol which lights up when selected. However, unique features specific to only this model include 2 detection jacks, 6 outputs, and a design tailored chiefly for clinics and hospitals.
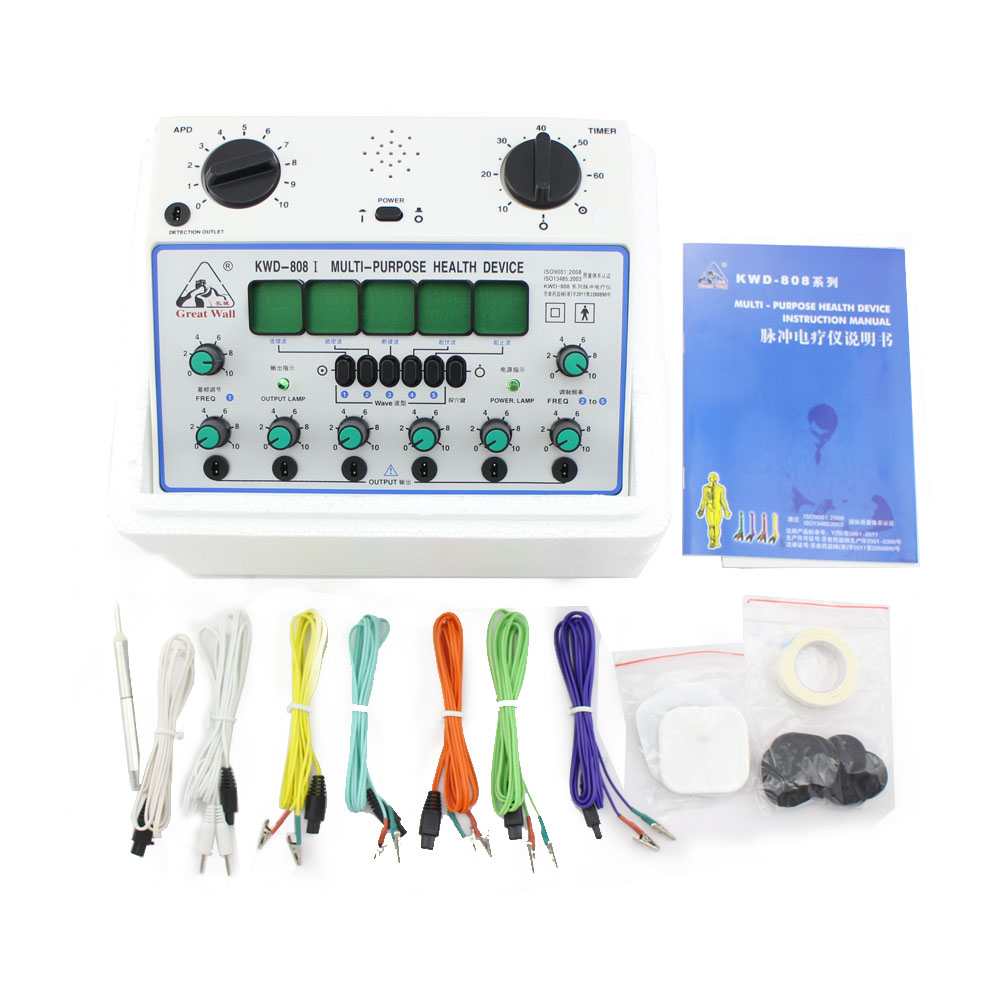
Electric Acupuncture Stimulator
Acupuncture Stimulator,Acupuncture Needle Stimulator,Tens Acupuncture Stimulator,Tens Needle Stimulator
Shenzhen Guangyang Zhongkang Technology Co., Ltd , https://www.lighttherapymachine.com